ISO 9001 vs GMP: Key Differences, Similarities, and Benefits
5 October 2023
ISO 9001 vs GMP is one of the most common comparisons in quality management. Both standards focus on product quality and customer trust, but they serve different industries and regulatory needs. Understanding the difference between ISO 9001 and GMP helps companies choose the right framework for compliance, certification, and customer satisfaction.
If your business needs help with ISO 9001 certification, you can explore our ISO 9001 Certification Toolkit. It includes everything you need to implement the standard effectively and save thousands in consulting fees.
What is ISO 9001?
ISO 9001 is the most widely used quality management system (QMS) standard in the world. Published by the International Organization for Standardization (ISO), it provides a framework for companies to improve processes, reduce errors, and increase customer satisfaction.
Key facts about ISO 9001:
Applicable to all industries
More than 1 million companies in 170+ countries are certified
Focuses on risk-based thinking, continuous improvement, and customer satisfaction
Not industry-specific, unlike GMP
ISO 9001 helps businesses gain global recognition, streamline processes, and meet customer expectations.
What is GMP Certified?
To understand what is GMP certified, you need to look at Good Manufacturing Practices (GMP). GMP is a system of regulations that ensures products are consistently produced and controlled according to quality standards.
GMP is usually enforced by government agencies such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA). Certification means a company complies with these requirements during production.
Industries covered by GMP include:
Pharmaceuticals
Food and beverages
Dietary supplements
Medical devices
Cosmetics
Being GMP certified means the company follows strict rules for hygiene, safety, equipment, materials, and documentation.
Learn more about GMP on Wikipedia.
ISO 9001 vs GMP: Core Differences
While both systems focus on quality, they differ in scope, purpose, and application.
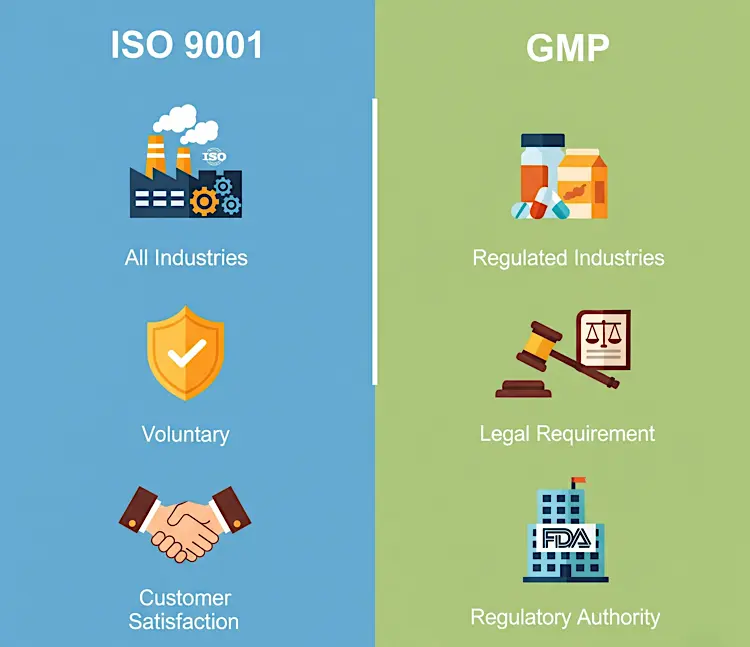
Industry Scope
ISO 9001's scope is broad, covering all industries, while GMP's scope is narrow, focusing on regulated sectors like pharma and food
Legal Requirement
ISO 9001 is a voluntary certification, while GMP is often legally required by governments
Certification Process
ISO 9001 is audited by accredited certification bodies, while GMP is audited by regulatory authorities such as the FDA
Focus
ISO 9001 focuses on customer satisfaction, risk management, continuous improvement, while GMP focuses on product safety, hygiene, regulatory compliance
GMP Quality Management System vs ISO 9001
The GMP quality management system (GMP QMS) is highly prescriptive. It includes mandatory procedures for sanitation, equipment validation, personnel training, and record-keeping.
ISO 9001, by contrast, is flexible. It sets principles such as leadership, planning, support, operations, and improvement but allows companies to adapt them.
Both systems require:
Documented processes
Internal audits
Corrective and preventive actions
Continuous monitoring
However, GMP QMS leaves less room for interpretation. For example, in pharmaceuticals, even minor documentation errors can lead to regulatory penalties.
Why Businesses Compare ISO vs GMP
Companies often ask about ISO vs GMP because they want to understand if both are needed. The answer depends on industry and compliance requirements.
A pharmaceutical company usually must comply with GMP by law, but ISO 9001 can improve overall efficiency.
A manufacturing company outside pharma or food will benefit more from ISO 9001, as GMP does not apply.
Some businesses choose both to combine regulatory compliance with international recognition.
The Role of QSR Quality
Another related concept is QSR quality, short for Quality System Regulation. This applies mainly to medical devices under the FDA in the United States. QSR shares many principles with GMP but focuses specifically on device safety and effectiveness.
Companies in this sector often integrate ISO 9001 with QSR to create a stronger quality culture.
Benefits of ISO 9001 Certification
ISO 9001 offers many benefits, especially for businesses outside regulated industries:
Global recognition and credibility
Better customer satisfaction
Reduced operational costs
Stronger risk management
Continuous improvement culture
If you want expert guidance, our ISO 9001 Certification Service provides full consulting and support.
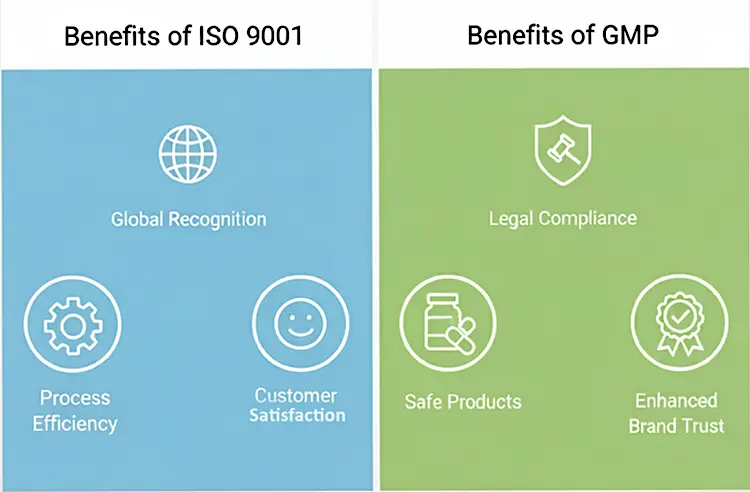
Benefits of GMP Certification
For companies in regulated sectors, GMP certification provides:
Legal compliance with FDA, EMA, and other authorities
Safe products for consumers
Stronger brand trust
Better access to international markets
Failure to comply can result in product recalls, fines, or even shutdowns.
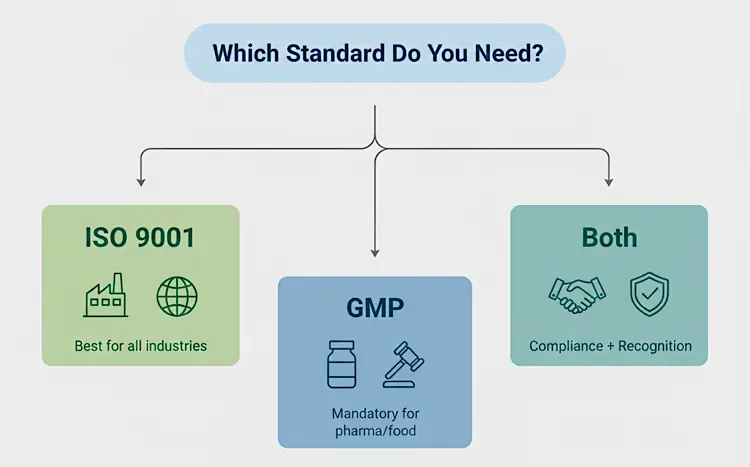
Do You Need ISO 9001, GMP, or Both?
The choice depends on your industry and goals:
ISO 9001 only: Best for companies in non-regulated sectors like IT, manufacturing, or services.
GMP only: Required for pharmaceutical, food, and medical device companies.
Both: Recommended if you want regulatory compliance plus international recognition.
Many businesses combine ISO 9001 and GMP to ensure compliance plus competitiveness.
ISO GMP Integration
Integrating ISO GMP requirements creates a comprehensive QMS that covers both compliance and performance. This approach avoids duplication and reduces costs.
For example:
GMP ensures safety and compliance
ISO 9001 ensures efficiency and customer satisfaction
Together, they provide a complete framework for quality excellence.
How to Get Certified
ISO 9001 Certification Steps
1. Gap analysis of your current processes
2. Documentation development
3. Implementation and training
4. Internal audits
5. External audit by a certification body
Our ISO 9001 Certification Toolkit provides a comprehensive and guided solution for stress-free certification.
GMP Certification Steps
1. Facility and process assessment
2. Compliance with hygiene and safety requirements
3. Documentation control
4. Staff training in GMP procedures
5. Regulatory inspection by authorities
Conclusion: ISO 9001 vs GMP
The ISO 9001 vs GMP debate is about scope and application. ISO 9001 offers global recognition and efficiency for all industries, while GMP is a legal requirement for regulated sectors. Many companies integrate both for maximum benefit.
If you want to simplify ISO 9001 certification, check our ISO 9001 Online Training. It helps managers and employees gain the skills needed to implement and maintain a compliant QMS.


